1. Post a picture of three 3-dimensional Ball and Stick
molecular models that you have created
with common items around your home. Also post a molecular structure image and the
IUPAC name of the molecule.
ANSWER:
#1

#2


ANSWER:
Here is a short list of some familiar molecules
- Chlorine
- Ammonia
- Ethanol
- Hydrogen Peroxide
- Water
- Ascorbic Acid (Vitamin C)
Carbon Dioxide= CO2
#2
#3
2. Post an image from the web, the chemical systematic
(IUPAC) name, common name, and the molecule formula for 20 chemicals that you
use or eat. Explore the ingredients of things like cosmetics and foods.
#1
Glucose (Sugar)
#1
Glucose (Sugar)

#2
Trimethylxanthine (Caffeine)
#3
Ethanol (Found in alcoholic beverages)

#4
Hydrogen Peroxide (disinfectant solution)

#5
Ascorbic Acid (Vitamin C)
#6
Acetaminaphen (Main active ingredient in Tylenol)
#7
Sodium Bicarbonate (Baking Powder)
#8
Sodium Hypchlorite (Bleach)
#9
Calcium Carbonate (Chalk)
#10
Glycerol (Glycerin soap)
#11
Carbon (graphite in pencils)
#12
Titanium Dioxide (Liquid Paper)
#13
Sodium Chloride (Table Salt)
#14
Sodium Fluoride (Toothpaste)
#15
Acetone (Main active ingredient in Nail polish remover)

#16
Aluminum (foil for preserving/ cooking/ baking)
#17
Trisodium (Artificial Coloring)
#18
Acetic Acid (Vinegar)
#19
Sulfuric Acid (Battery acid)
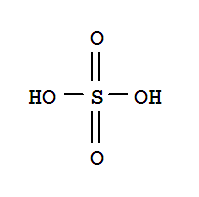
#20
Water Oxidane (water)
3. Look over your molecules and the bonding characteristics,
how many bonds does each of the following elements typically have? Carbon? Hydrogen? Oxygen?
ANSWER:
Carbon: 2 for Carbon Dioxide
Hydrogen: 2 for Water and 2 for Hydrogen Peroxide
Oxygen: 1 for Water and 2 for Hydrogen Peroxide
ANSWER:
Carbon: 2 for Carbon Dioxide
Hydrogen: 2 for Water and 2 for Hydrogen Peroxide
Oxygen: 1 for Water and 2 for Hydrogen Peroxide
4. What does IUPAC stand for?
ANSWER:
IUPAC means: International Union of Pure and Applied Chemistry
ANSWER:
IUPAC means: International Union of Pure and Applied Chemistry
5. As you explore ingredients, notice how everything around
us is made up of chemicals consisting of atoms bound together into
molecules. But what about companies that
claim their products are chemical free! How can this be? Here is an example:
http://www.naturalhealthcareproducts.com/Cleaning-Products.php
Do a little web searching and propose what chemicals are actually in this product. Keep in mind, that everything at the molecular level is a chemical, whether it be made in nature or in a lab.
ANSWER:
After reviewing the above listed web address, I have concluded that there is no possible way for any of these so-called "chemical free" products to NOT contain some sort of chemical. Although they may be using safer chemicals, there is still molecular structures being used (i.e. chemical formulas). Therefore, we can assume that all the hype related to "chemical free" products for cleaning is more about environmentally friendly cleaning solutions that include less harmful chemicals.



No comments:
Post a Comment